Effectively Controls Bleeding for Percutaneous Catheterization
SyvekExcel® and SyvekNT® contain poly-N-acetyl glucosamine (pGlcNAc) fibers derived from microalgae. The fibers from this marine polymer are proven to accelerate platelet activation, red blood cell aggregation and vasoconstriction.1 In a randomized, double-blind, placebo-controlled study, pGlcNAc applied to the patients' femoral access site significantly reduced time to hemostasis.2 SyvekExcel and SyvekNT are non-immunogenic with no known contraindications.
The Unique Structure, Dimensions, And Physicochemical Properties Are The Keys To The Efficacy Of Syvek®.3
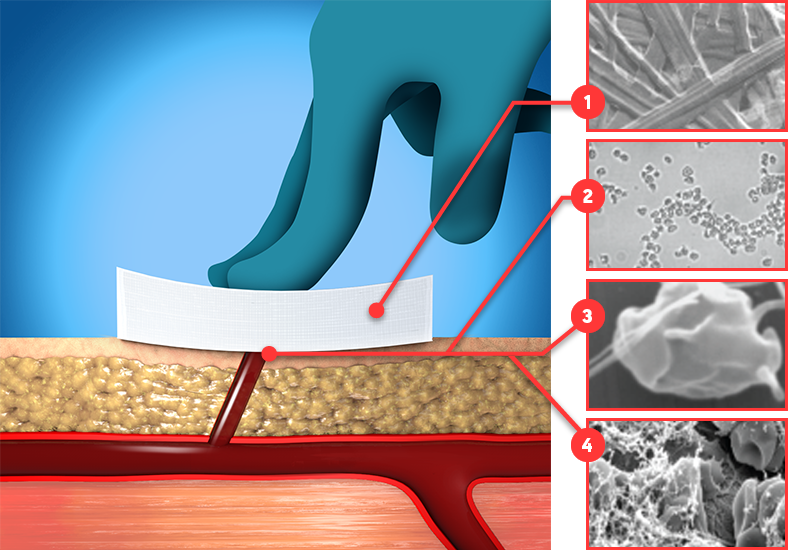
When blood contacts pGlcNAc, plasma proteins are rapidly bound and adsorbed.3
Fibers in the matrix interact with platelets, stimulating their activation leading to the onset of the coagulation cascade.3
A catalytic surface for thrombin generation and accelerated fibrin clot formation results from the interaction of platelets with pGlcNAc.4
The fibers bind and cause agglutination of RBCs, resulting in the exposure of phosphatidylserine, leading to their activation and direct participation in clotting.3,5
The combination of platelet and RBC receptor-based contact with the pGlcNAc fibers results in thrombin generation and fibrin mesh formation. A hemostatic plug forms, which is augmented by additional vasoconstrictive effects due to the release of both thromboxane by activated platelets and endothelin-1 by endothelial cells.3
Effective for a Variety of Procedures and Patients
The poly-N-acetyl glucosamine fibers present in SyvekExcel and SyvekNT effectively control bleeding for patients undergoing a variety of diagnostic and interventional procedures including femoral and radial catheterization. Syvek is equally effective in treating patients with medically and genetically induced coagulopathies and hemorrhage – even with activated clotting times (ACTs) up to 300.6 Syvek can be used with various sizes of catheters as well.
Control Bleeding with a Minimally Invasive Approach
As externally applied products, SyvekExcel and SyvekNT may provide distinct advantages over subcutaneous closure devices such as plugs. These closure devices preclude reintervention at the same site for extended periods of time. Because Syvek leaves no foreign materials behind at the puncture site, it allows the surgeon to easily reaccess the same artery for secondary or follow-up procedures.7
To learn more about the control of bleeding using SyvekExcel and SyvekNT, please contact us today.
Indications for Use:
SyvekNT® is intended for the promotion of rapid control of bleeding in patients following hemodialysis and in patients on anticoagulation therapy. SyvekNT is intended for use in the local management of bleeding wounds such as vascular access site, percutaneous catheters or tubes and surgical debridement. Syvek Excel® is intended for use following femoral vascular catheterization procedures to assist in obtaining and maintaining hemostasis.
Safety Information:
PRECAUTIONS: There are no known contraindications to the use of SyvekNT®. Longer compression may be necessary for hypertensive or obese patients. There are no known contraindications to the use of Syvek Excel®. Longer compression may be necessary for hypertensive or obese patients.
Not made with Natural Rubber Latex
CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician or other licensed practitioner.
Manufactured by Marine Polymer Technologies, Inc. For instructions for use or additional product information, please contact us.
- 8,992,453
- 8,859,528
- 8,835,408
- 8,834,516
- 8,481,512
- 8,152,750
- 7,931,637
- 7,115,588
- 7,041,657
- Jewelewicz D, Cohn S, Crookes B, et al. J Trauma. 2003;55:275–281.
- Najjar SF, Healey N, Healey CM, et al. Am J Cardiol. 2003;92(Suppl):151L.
- Valeri C, Vournakis J. J Trauma. 2011; 71:S162-166.
- Fischer TH, Thatte HS, Nichols TC, et al. Biomaterials. 2005;26:5433-5443.
- Scanning Electron Micrograph courtesy of Thomas H. Fischer, PhD. Dept. of Pathology and Laboratory Medicine. University of North Carolina, Chapel Hill, NC.
- Nader R, Garcia J, Drushal K, et al. J Invas Cardiol. 2002; 14:305-307.
- Hirsch JA, Reddy SA, Capasso WE, et al. Tech Vasc Interv Radiol. 2003;6:92-95.
